Antigen-antigen receptor interaction
Antigens interact with antigen receptors on the basis of complementary shapes and intermolecular forces. They do not bind covalently but by protein-protein interactions.
Intermolecular forces - Forces that act between stable molecules or between functional groups of macromolecules. Intermolecular forces (the weakest of which are van der Waals forces) include momentary attractions between molecules, diatomic free elements, and individual atoms.
The 5 kinds of protein-protein interactions:
- Hydrogen bonding - Chemical bond which arises from the attraction between the slight positive charge on a hydrogen atom and a slight negative charge on a nearby fluorine, oxygen or nitrogen atom. Weak bonds, but found in great quantities in proteins, nucleic acids, and other biological macromolecules.

- Ionic interactions - Chemical bond formed by electrostatic attraction resulting when one atom “donates” valence electron(s) to another atom, resulting in filled energy shells for both atoms involved in the interaction.

- Hydrophobic interactions - An interaction between a hydrophobic (''water-hating'') part of a molecule and an aqueous environment
- Dipole-dipole - The interaction of two atoms, molecules, or nuclei by means of their electric or magnetic dipole moments.

- Van der Waals - The attractive or repulsive force between molecules (or between parts of the same molecule) other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral molecules.


Covalent bond - A form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms, or between atoms and other covalent bonds.
Cross-reactivity
Cross-reactivity - The binding of antibody to similar epitopes on other antigens / proteins.
For examples, when an antibody bings more strongly to say, antigen A than to antigen B, it is said to be more specific for antigen A compared to Antigen B.
The binding strength of antibodies to antigens depend on two factors:
- Antibody affinity - Strength of binding of an antibody binging site to an epitope. It is also the sum of all the attractive and repulsive forces. It depends on opposing atomic groupings and the shape of combining site to the epitope.
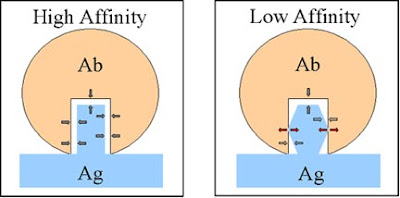
- Antibody avidity - Total strength of binding between a miltivalent antigen and a multivalent antibody. It is also the sum of individual binding affinities.

Multivalent - Having several sites of attachment for an antibody or antigen.

References:
http://student.biology.arizona.edu/honors2004/group03/Vocabulary.htm
http://www.answers.com/topic/dipole-dipole-interaction
http://www.abcam.com/index.html?pageconfig=resource&rid=11279&pid=11287
http://www.st-andrews.ac.uk/~ulf/levvdw.gif
http://upload.wikimedia.org/wikipedia/commons/5/59/Dipole-dipole-interaction-in-HCl-2D.png
http://chemtools.chem.soton.ac.uk/projects/emalaria/page_imgs/content/ionic.gif

No comments:
Post a Comment